Phase I and Experimental Medicine Studies Governance Standards Framework

Co-authors:
Saul Faust, Chris Edwards and Kim Lee, NIHR Southampton CRF
Richard Fitzgerald and Jennifer Gibney, NIHR Liverpool CRF
ENHANCED GOVERANCE STANDARDS
The NIHR Directors Group have developed two new documents to assist the delivery of phase I and experimental medicine clinical trials:
- Phase I and Experimental Medicine Governance Standards Framework: The purpose of this framework is to provide a guide to the enhanced governance processes including risk assessment that will allow all CRFs to work to a minimum agreed standard for the highest risk studies.
- Risk Stratification Matrix and Contingency Plans: The Risk Matrix has been developed as a tool to risk assess a Phase I classified or experimental study and allow pragmatic and risk-adapted approach to manage the possible risks to patient safety associated with the trial. This document can be completed by one site and shared with all others involved.
BACKGROUND
Following the TGN1412 incident in March 2006 the MHRA developed a voluntary ‘Phase I Accreditation Scheme’ for research facilities who conduct Phase I clinical trials.
It was also recognised by the UKCRF Network Directors that it is in the best interest of patient safety across all CRFs and the wider NHS to establish a framework for facilitating the highest risk early phase and experimental studies.
This framework has now been developed and has a similar approach to the MHRA Phase I scheme of; risk assessing studies, implementing where necessary additional procedures to the requirements outlined in the protocol forming the overall contingency plan for the study dosing visit, as well as other requirements.
WHAT DOES THE FRAMWORK INCLUDE?
The framework outlines areas of the formal MHRA scheme that should be adopted as best practice for all CRFs for the highest risk early phase and experimental medicine studies. The document also describes a governance framework, under which the highest risk studies should be managed. These principles can be applied in other NHS organisations that do not currently have a dedicated CRF.
The framework covers the following procedures:

- Clinical Trial Design and Set-up
- Medical Emergencies and Facilities
- Staff
- Subject Recruitment Identification
- Quality Management / Quality System
The framework also includes an example Early Phase Safety Committee terms of reference and a dose escalation decision tree.
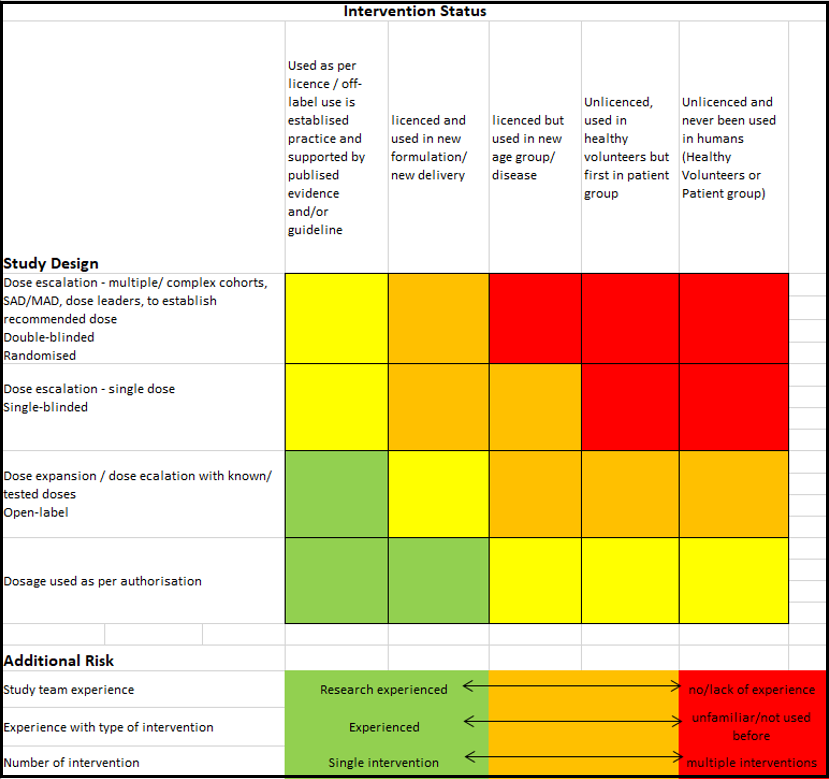
RISK STRATIFICATION MATRIX
The matrix provides a risk outcome based on the intervention status (e.g. ‘licenced but used in a new age group/disease’), study design, and additional factors (i.e. study team experience). It is the responsibility of the PI to make these assessments, and where necessary, peer reviewed to ensure the outcome reflects the study conducted at their site.
For studies with a Low or Medium risk outcome, the corresponding pre-set contingency plan will be used. All three contingency plans outline the same additional procedures to increase the patient safety. Depending the risk matrix outcome, the additional procedure may either be not or must be implemented. It is also indicated on certain contingency plans if it’s the PI decision to implement the procedure as they see fit. For studies with a high risk, an additional risk assessment will be required to identify the risk and the mitigation plan.
WORKING TOGETHER
In cases where multiple facilities are conducting the same study, the risk stratification matrix template can be completed and shared across all sites. This process was successfully piloted in preparation for the valneva vaccine study in December 2020 and will also be used across the Network for the AGILE study in the coming months.
The Network will be collecting feedback and reviewing the document later this year, so please send any comments or questions to [email protected]
HOW CAN THE DOCUMENT BE ACCESSED?
The phase I governance framework and risk stratification matrix form part of our new Phase I Toolkit that can be downloaded from the UKCRF Network Portal. The toolkit also includes existing tools developed by the Network: Dosing Day Checklist and Emergency Scenario Training Guidance.
If you are not already a user, complete the request form at the bottom of the Portal page to gain access.
AKNOWLEDGMENTS
The documents were developed by:
- Saul Faust, Chris Edwards and Kim Lee, NIHR Southampton CRF
- Richard Fitzgerald and Jennifer Gibney, NIHR Liverpool CRF
Reviewed for UKCRF Network by:
- Vincenzo Libri, NIHR UCL CRF
- Phil Chowienczyk, NIHR Guys and St Thomas’ CRF
- Dave Newby and Steve Cunningham, Edinburgh WTCRF (for Scotland/Devolved Nations)
The risk assessment matrix was developed from the current processes at NIHR Royal Liverpool and NIHR Southampton, which were informed by previous work undertaken in Edinburgh.